Corrosion is degradation by a chemical reaction on stainless steel, which depends on its surrounding environment.
Stainless Steel usually has a 2-3 nanometer thick passive layer of chromium on its surface which rebuilds if mechanically damaged. This layer protects the stainless steel from corrosion. However, sometimes the environment is unfavorable, and stainless steel can corrode. There can be various kinds of corrosion and can be prevented if we analyze the factors and environment responsible for its formation. The most common types of corrosion are as below :


Uniform Corrosion
This is the most common type of corrosion found in areas where steel surface is exposed to open atmosphere, soil, and natural waters leading to a rusty appearance. The corrosion spreads evenly on the surface by oxidation reaction.
Prevention : Use thick materials, coating the surface with paint, galvanizing, or anodizing.
Pitting Corrosion
Occurs on metal surfaces as small diameter holes on the object’s while the other parts of the metal surface remain unattacked. Pitting originates in the areas where inconsistencies in the passive protective film exist. This may be due to film damage, poor coating application, or foreign deposits on the metal surface. Pitting tends to penetrate the thickness of the material.
Prevention : Add Cr(chromium), Mo (Molybdenum), N(nitrogen), PRE (Pitting Resistance Equivalent) value should be higher.


Crevice Corrosion
Crevice Corrosion is also a penetrative type of corrosion that occurs in or directly adjacent to gaps on the surface of a metal. When two metals or metal with a nonmetal are connected, and there is an accumulation of dirt or deposits, the corrosions occur in the crevice, and surrounding areas remain unaffected. This type of corrosion is also observed in the regions where some fluid Is stagnant. Due to lack of movement, a positive ion is abundant in the crevice, leading to electrochemical reactions and acidic in nature. This acidic fluid breaks the passive layer and results in dangerous corrosion.
Prevention : avoid the accumulation of chlorides, salts, and pollutants
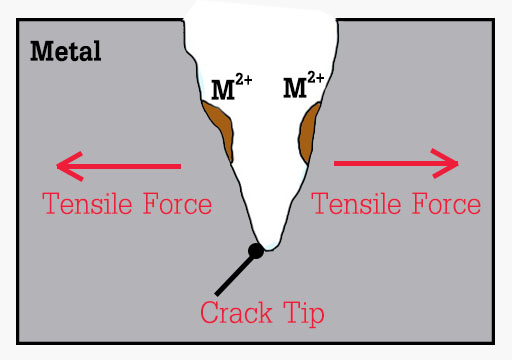
Stress Corrosion
Stress corrosion is when the metal is more susceptible to stress. Corrosion is faster if the steel is more prone to tensile stress. Sometimes fine cracks are observed at the metal surface. These cracks are only at the specific areas on the surface due to the presence of tensile stresses in the corrosive environment.
Prevention : Control operating temperature and the electrochemical potential, control hardness and stress level.
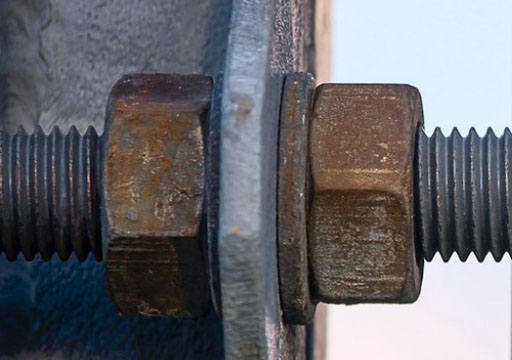
Galvanic Corrosion
Galvanic corrosion may occur when two different metallic materials are electrically connected in a corrosive environment. If we use two similar kinds of SS grades, there will be no galvanic corrosion but if one of the metals is carbon steel and one is stainless steel, then corrosion will occur due to a difference in electrode potential.
Prevention : Use similar types of noble stainless steel.
Erosion Corrosion
This type of corrosion is usually seen where there is fluid flow. The fluid continuously flows, damages the protective layer on the stainless steel, and quickly causes corrosion.
Prevention : Reduce turbulence and control fluid velocity.


Intergranular Corrosion
Stainless steel usually contains chromium on its surface to protect it from corrosion. During the welding process, the steel undergoes elevated temperatures of 500°C to 800°C, which reduces chromium grains from the surface. This reduction results in corrosion and is called Intergranular corrosion.
Prevention : Use low carbon grades (304,316), grades with titanium, and post-weld heat treatment.
Atmospheric Corrosion
Usually occurs on fences, railings, or gates exposed to the humid atmosphere or at high temperatures. This corrosion may also be seen on steel with rough surfaces. However, Stainless steel grades 304 and 316 L are commonly used at the beachside to avoid corrosion.
Prevention : Use SS grades 304 and 316L.

In conclusion, we can avoid corrosion primarily if we choose the “right” grade, study the environment carefully before any application and always remember that chlorides are harmful and lead to corrosion!
References:
- Introduction to Materials and Engineering by Pearson
- materials.sandvik
- corrosionpedia.com
- corrosionclinic.com


